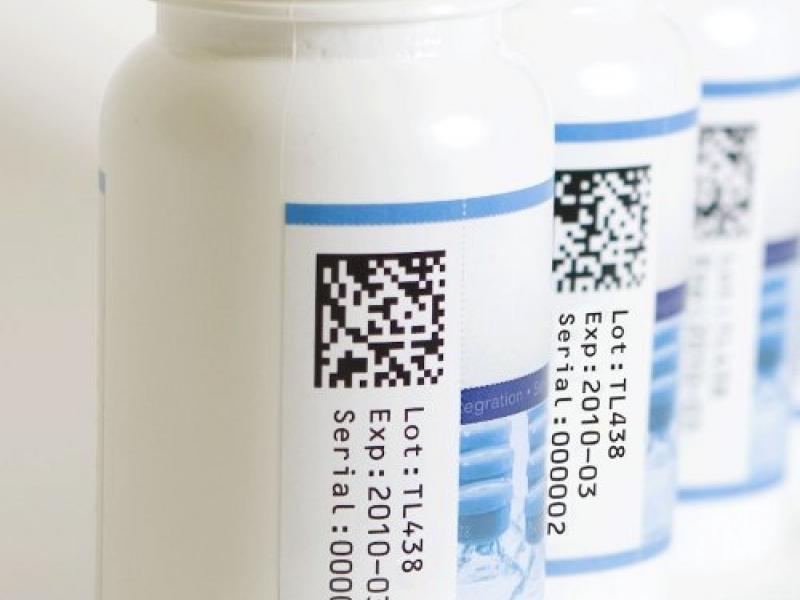
In an effort to combat the world of counterfeit drugs and keep patient safety top of mind, The Federal Drug Administration enacted the Drug Supply Chain Security Act (DSCSA), a phased approach to pharmaceutical serialization. By November of this year, pharmaceutical manufacturers must include a unique product identifier on prescription drug packaging and cases. Read the full article…



